The last draft post I wrote about the MEGA petition was superseded by events before I finished it, so I’ll try and crack on with this one before the same thing happens again. Of course ‘cracking on’ in ME terms is still kind of slow but I’ll see if I can break the tortoise barrier.
So, what’s happened recently?
Well, we’ve been told that Peter White is retiring from research and will only be an ‘advisor’ to MEGA from now on. This perspective appears to be endorsed by the latest list of MEGA personnel, which no longer includes him. I can only give a muted ‘hurrah’ to this one. Advice is dangerous stuff and you can still do a lot of damage with it. His PACE Trial is swiftly becoming a watchword for bad science (see here, here, and here). Is he really the sort of ME ‘expert’ that either we patients or the MEGA team want around to guide this latest project?
It really is astonishing that MEGA apparently do still want him around after all he has done, and that they clearly expect patients to put up with it. It seems to me that if a passing Martian was given a brief course in English and the full facts, then even he (or she) would swiftly understand why we don’t want Prof White anywhere near this project. Why do the MEGA team not get this?
People with ME have been left on the scrapheap for decades. I myself have been ill for over thirty years. That’s over half my life. I have no children because of it. I lost my job. My life is very limited. Yet I am one of the relatively lucky ones. I can sit and tap at this keyboard – as long as I take plenty of rests to fend off the shoulder and eye pain and overall exhaustion. There are plenty of others who have to spend all their lives in bed, who can’t stand the light, who can’t even talk to their loved ones. We’ve all heard about Whitney Defoe whose birthday it recently was. He is not alone in his suffering. The vast majority of the severely ill are left to fend for themselves as best they can. Rarely do doctors come near them and they wouldn’t know what to do if they did.
And all this time, all these decades, so little research has been done, in large part because of the fairy story dreamed up by the PACE researchers and their associates: the fairy story that all we have to do is cast away our fear and we can get better again. In their attempts to give credence to this pipe dream, they have sucked up the majority of what little research funding there has been for ME in the UK and channeled it into their largely inconclusive projects, testing on populations with fatigue conditions other than ME and even then achieving unconvincing results – foremost among them PACE itself, which has now been exposed as a sham, even the modest results it claimed revealed to be dodgy statistics and sleights of hand.
Why is it so hard for those in MEGA to understand why we feel so strongly about Peter White and his friends? Now at last we are getting some biomedical research and they want him in on it. Why? How can they expect us to trust him?
This graphic (produced by Action for ME) illustrates precisely to whom the UK research money has gone in recent years (2006 to 2015), and those getting the most funding have not been doing biomedical research:
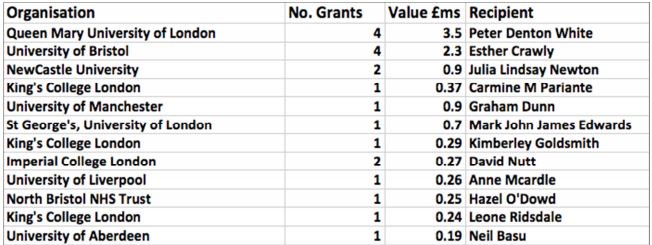
Two names predominate: Peter White and Esther Crawley, and the latter seems to me to be just as much of a liability for MEGA as is White.
Earlier this year, you may remember, Esther Crawley published a study suggesting that the prevalence of (as she described it on BBC Radio 4) CFS/ME at age 16 was higher then previously considered. For this analysis, she relied on children and their parents filling in questionnaires about whether they felt tired or not. There was no input from a doctor and no attempt to screen out patients with other fatigue-related conditions (other than depression, which was once again assessed from questionnaire results). The paper reads: ‘it is important that the uncertainties regarding the population prevalence of pediatric CFS are resolved’ yet it seems unlikely that a study involving such haphazard self-assessed inclusion criteria could have done anything other than add to the confusion. It would seem to me to be very unfortunate for a researcher who seems to have such a poor grasp of the need for carefully defined criteria to be involved with patient selection for MEGA.
A few days ago, at the CMRC conference, she was seeking to justify her trial of GET for children by saying there was ‘good evidence’ for its use in adults. I presume she meant the PACE trial. She does not seem to have kept abreast of recent developments.
But enough of the personnel. We have made it very clear why we don’t want Profs White and Crawley involved with the project but I think there other issues with it which are just as important.
The latest MEGA update, which appeared a few days ago, made it clear that patients for the study would come from the English NHS clinics, which diagnose using the NICE criteria. I have to admit to a sinking feeling when I heard this, one which has stayed with me ever since.
To put it baldly, this will not produce a representative sample of people with ME/CFS. There are a number of reasons why:
- Most severely affected people are unable to access the NHS clinics. A few are reached by home visits or telephone/Skype consultations but most do not have this opportunity. Is it really intended that they should not be included in this massive project? The material accompanying the MEGA petition reads: “We want to investigate not just the causes and underlying biology of M.E./CFS, but also its different types (sub-phenotypes) which may be caused by different underlying mechanisms. This may eventually enable development of new diagnostic tests and new treatments for each sub-phenotype.” Is it not likely that the severely ill have important data to provide towards this end? Prof Ron Davis has said that severely affected patients provide the most useful data “because their biology would show the greatest differences compared with healthy controls”. Is this opportunity to be lost to MEGA – and are the severely affected to be ignored yet again, even as £5m or more is invested?
- The plan to use other diagnoses subsequent to NICE is unsound. The same sort of error was made in the PACE trial. The patients will have been initially diagnosed using the NICE criteria and subsequently will be filtered with others. But any patients who didn’t fit NICE but might have fitted one or more of the other criteria will be lost to the project. It is wrong to assume you have everyone covered just because NICE is a broad criteria.
- Now we get to the tricky bit. It is controversial but there’s no way to ignore it. The clinics select their patients with the treatments they have to offer in mind. This will inevitably entail a bias in selection. They will be more likely to diagnose patients with CFS/ME if the clinician feels they will respond well to GET or GAT. We need to remember that they have one eye on their outcome figures and are under pressure to perform well in comparison with other clinics. The bias may not be a conscious one but it is a bias nevertheless and it is surely appropriate to take this sort of bias into consideration in research (in the same way as the desirability of double blinding for instance). Steps will need to be taken to correct the effect of the bias.
- There is also a problem, which I suspect is widespread, that patients who do not respond well to the therapies (or refuse them in the first place) and therefore drop out are not necessarily followed up by the clinics. These are likely to be genuine ME patients rather those who might have CFS. Unless they can be chased up and brought into the study, this will be another factor skewing the sample of patients.
If the will is there, it should be possible to correct these various ‘skewing factors’ (I expect there’s a proper word for that but I’m not a statistician!) by reaching out to other patients in addition to those provided by the clinics. Prof Julia Newton, with the assistance of Victoria Strassheim (who also spoke at the CMRC conference the other day) placed adverts in ME support group magazines and social media etc to find patients for their own severely affected study. Similar measures (perhaps after consultation with Newton and/or Strassheim) could be taken by MEGA to find not only severely affected patients but also additional patients to make up for those who did not fit the NICE criteria and those who were lost by the inevitable bias mentioned earlier.
I feel it is also important that MEGA reaches out to children in the same way so that not all them come from the Bristol clinic. Once again, it is a question of balance and diversity to build up a representative cohort. Perhaps they could come from Scotland and Wales, and perhaps Jane Colby would be willing to help to ensure that severely affected children are represented.
An additional issue is that there are numerous references throughout the latest MEGA update to having to limit the amount of data collection if finances don’t allow or the patient advisory group objects. The issue seems to me to be overstated and I’m concerned it might serve as an all too useful excuse for not developing a Canadian Criteria cohort, which I feel is vital to the success of the study. My fears in this respect are amplified by the knowledge that Profs White and Crawley have already made it clear that they consider the Canadian criteria to be ‘not practicable’.
It’s also worth pointing out that diagnoses in the NHS clinics are usually made by triage involving OTs, nurses etc rather than by doctors. Many of these may be good clinicians but others will inevitably be inexperienced and may only have picked up what they know about ME/CFS from reading the clinic’s own leaflets. Some of them will have a background in mental health. It seems likely therefore that they will concentrate on what they believe to be the principal symptoms of ME rather than taking a full medical history as the MEGA update suggests..
Finally I’d like to make it clear that, unlike some patients, I’m not against the clinics per se. The ones which teach graded activity (as opposed to exercise) seem to me to be helping people to pace. They may also employ CBT to assist people in coming to terms with having the illness rather than (as in PACE) trying to brainwash patients into thinking they’re not ill. Many people find these clinics helpful. Other clinics unfortunately use graded exercise but I’m fortunate enough not to have direct experience of any of those.
However, I don’t think, as I’ve explained, that the clinics will automatically provide a representative sample of patients for MEGA. Steps will have to be taken to redress the balance and there will need to be a will to take those steps. To judge from the latest update, objections will be made on the grounds of cost, to which, I think, our rejoinder must be: if you can’t afford it, do something cheaper. Whatever is done, it needs doing properly. If the patients are taken only from the NHS clinics, the sample will be skewed towards CFS rather than genuine ME, and MEGA will be another PACE around our necks.
I intend to write along these lines to whoever seems appropriate. My wife has already sent such a letter to Dr Charles Shepherd of the ME Association who has promised to bring all patient concerns to the appropriate people on the MEGA team.He has also suggested that people send feedback to Prof Stephen Holgate, Chair of the CMRC. I will also write to Sonya Chowdhury of AfME (who represents the patient charities on the MEGA team) and ME Research UK.
The above are just my ideas. I’m sure there are many other issues I haven’t even thought of. Over to you! I must say that working through them all seemed a bit like working through the faults in PACE, which is not an encouraging thought..

A great blog with some excellent observations.
I completely agree with your concerns about selection bias in the NHS CFS Clinics. My own experience would support what you say. Unfortunately, the BPS model is still all-pervading within the NHS approach to ME/cfs (fear of repercussions makes it virtually impossible for doctors to do otherwise) and so it will be difficult to get away from that in any research which recruits participants via their structures.
Regarding PDW’s retirement from research, I think that to say he is retiring from all ME/cfs research isn’t yet established is it? Yes, he won’t be actively involved in MEGA, but I fully expect him to continue to churn out more PACE spin-off garbage in an attempt to shore up the crumbling edifice of his life’s work.
More worrying, is the thought that he is freeing up time to enable him to devote to the NICE guideline review which is scheduled to begin next year. He must be prevented from being directly involved.
LikeLiked by 2 people
Thank you. Yes, I suspect Peter White’s retirement can be viewed in the same way as Simon Wessely’s claim to have withdrawn from the ME field because of all the death threats. As you say, he will probably get further mileage out of PACE and there will most likely still be journals foolish enough to publish it. Then there’s the NICE guideline review and the MEGA project… I can’t see him staying out of them even if it’s behind the scenes. By any rational standards, he should be an outcast with his reputation in tatters after PACE but the medical profession doesn’t seem to work like that.
LikeLiked by 2 people
I think you have a good point here. I would be a specific example. If well (I’m not) I could walk to the main study I’m so close. I also fit the various case definitions, had viral onset, and my sister also had severe ME (so I likely contain some useful epigenetic and genetic info if you look in the right places). Despite being 95% housebound I could actually access this study without them having to do a home visit to get my data. However, when I read how they’re recruiting I realised that I won’t be recruited for this, unless they adjust criteria.
My GP and I gave up on the NHS centre years ago in terms of them being able to offer any additional help (it was some use for activity management but they can’t really be called treatment centres, I never saw a doctor, it is more like limited training). This means that I won’t be on their list.
I’m currently sick on Sick so not up to writing something formal right now, but when I do I think I’ll suggest that GPs might be a better avenue for a broader sample. They will have a much better idea of who has severe ME for example. Patient groups are another avenue, but they could introduce different biases (people who join will be different from those who don’t, though perhaps in an opposite way to the Centres’ patient lists, both might be a good balance).
I wonder how you get to join the patient advisory group and if you have to be well enough to actually attend meetings?
I think getting the tone of our letters right is going to be tricky. Trying to communicate how much we want biomedical research whilst questioning every detail they suggest may come across as weird. Perhaps it could come across as passion for the importance of research design combined with emphasis on how as patients we cannot afford the time delay of questionable results again? I don’t think researchers are used to this type of scrutiny from patients. Many of us have the nonformal equivalence of research methods Masters via the PACE fiasco.
LikeLiked by 2 people
Hi Jenny – You say “I wonder how you get to join the patient advisory group and if you have to be well enough to actually attend meetings?” I think that’s something we have to find out. We really need people who understand the complex picture but it’s hard when we’re all so ill. The MEGA team will be looking for more compliant patients, I presume, probably ones who are not (in comparison) very ill and have done well on their therapies. As you say they are not used to this type of scrutiny so they will try to choose patients who don’t give them a hard time. As for selecting a wider range of patients, I will be suggesting they follow Julia Newton’s model but we don’t have to all make the same suggestions. Going through GPs is another valid idea.
LikeLiked by 2 people
I think you raise many valid points. There are so many things to consider in terms of designing a trial that we could support. I agree with you – the one thing that is overwhelmingly clear is that the inclusion of ANY of the PACE trial researchers on the research team ( even in an advisory capacity ) is totally unacceptable.
I also share your reservations about the plan to recruit patients from the “fatigue” clinics – this will exclude a great many people and as you say will immediately introduce a bias.
LikeLiked by 2 people
I agree with you all 👏💑 they are not looking at the bigger picture with ME 😢 💔. I do “NOT TRUST” in there Words to help #pwME.
LikeLiked by 2 people
Another nicely measured assessment, and yet more interesting articles linked that I hadn’t seen. Great to see the ripples spreading.
The first of your ‘here, here, and here’ links doesn’t seem to go to a PACE piece today–from my phone at least. The last ‘here’ to the clinical journal is particularly good.
LikeLike
Thanks, Steve. Re the first ‘here’ link, the linked article does mention PACE but only in passing. You need to look near the end, just past the subtitle ‘The Final Analysis’. It says: “But Levin says in no way do the study’s shortcomings rise to the level of fundamental errors of the kind he recently helped expose in the PACE trial for myalgic encephalomyelitis (a.k.a. chronic fatigue syndrome).” What is heartening (from the point of view of those of us who value the truth) is that PACE is starting to be used as a standard reference point for bad science in articles that are otherwise nothing to do with it.
LikeLike